|
|
Kezdőlap-Home
Page |
|
|
II. évfolyam 3. szám 2001. július [HUN] - Magyar cikk
|
Carbon Micro-Tubes Produced by Electrochemical Synthesis from Molten Salts M. S.Yaghmaee1, E. Cserta2, A.Kovács3, M. Árk4 1,2,4 LIMOS R&D, Department of Physical Chemistry, 3Department of Physical MetallurgyFaculty of Materials and Metallurgical Engineering, University of Miskolc, 3515 Hungary, Miskolc, Egyetemvaros
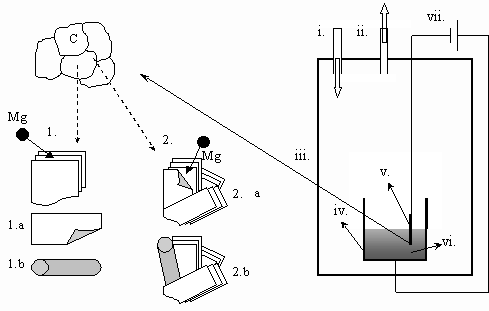
1. Introduction Carbon nano-tubes were discovered in 1991 by Iijima [1]. Since then, several methods of their synthesis have been described [2-5], such as evaporation of carbon in arc discharge, laser ablation, catalytic chemical vapor deposition of carbon containing gases and catalytic decomposition of fullerens. One of the most perspective ways to produce carbon nano-tubes in bulk quantities is the electrochemical deposition of liquid alkali metals from molten alkali halides onto graphite cathodes, discovered in 1995 by Hsu et al. (by the ‘Kroto-group’) [6-7]. These results have been extended by Chen et al (‘the Fray group’) [8-9]. In Hungary, carbon nano-tubes are mainly studied in KFKI, Budapest, by Bíró, Gyulai, and colleagues [10-11] (see also the review paper on Nanoscience of Gyulai [12]). The second effort on synthesis of carbon nano-tubes in Hungary is in progress in the framework of cooperation between the LIMOS R&D Group at the University of Miskolc (lead by G.Kaptay), where the synthesis was carried out from molten salts, and between the group at KKK (lead by E.Kálmán), where the nano-characterization of the samples is being performed [13-15]. During this cooperation it has been noted, that during the electrochemical synthesis of carbon nanotubes from molten salts carbon tubes with a very wide range of diameter are actually formed, scaling from several nanometers to several micrometers [13]. As carbon microtubes are somewhat closer to the engineering scale of our days, we have decided to carry out a research project on synthesis of carbon microtubes from molten salts. In the present paper our first, preliminary results will be demonstrated. 2. Experimental Conditions The electrochemical system used for the synthesis of carbon microtubes is shown in Fig.1. As a source of carbon, a graphite cathode of cylindrical shape was used, immersed into a molten salt mixture of 95 % NaCl and 5 % MgCl2. NaCl served as a solvent, while MgCl2 was chosen because the atomic radius of Mg is almost identical with the distance between graphite plates, and thus it was expected to give a highest yield of large carbon tubes. Three independent synthesis experiments were performed in order to check the reproducibility of results. The salts were first dried for 24 hours at 100 oC under vacuum. After drying the system was filled with Ar and its pressure was kept at 1.2-1.25 bars during the whole time of the experiment. The temperature of the system was increased to 850 oC and the salts were melted. After that the cathode (a graphite rod of 6.1 mm in diameter) and the reference electrode (a glassy carbon rod) were immersed into the salt to the depth of about 5 mm, and a cyclic voltammetric curve was taken by a computer controlled potentiostat. The electrolysis was carried out by applying the potential corresponding to the value of Mg deposition potential (about 1,100 mV in this system), in order to make sure that Mg is the only product deposited (note: in previous works the current was kept constant [13-15]). The electrolysis was performed for 10 min and after that the cathode was removed from the molten salt and the system was cooled down to the room temperature. Then, the solidified salt was dissolved in distillated water and mixed with toluene. Black, carbonescous particles were found sedimented to the bottom of the water phase and kept at the top, at the interface of the water and the toluol phases. Microtubes were found only in the sedimented fraction (see Fig-s 2-3), and on the cathode (Fig.4). The samples were examined by SEM.
Fig.1. Schematic representation of the electrolysis equipment and the two possible mechanisms of the growth of carbon nano/micro-tubes. i.: Ar input, ii.: vacuum output, iii.: stainless steel cell, iv.: crucible (used as the anode), v.: graphite cathode, vi.: molten salt, vii.: DC generator connected to a potentiometer 1.: intercalation, then banding in the molten salt (see Figs.2-3), 2.: intercalation, then banding and growth on the cathode (see Fig.4))
3. Experimental findings The SEM photographs of the sedimented in the water samples of the cathode can be seen in Figs.2-4. As one can see in Figs 2-4, micro-tubes were indeed formed during the process. They can be described as follows:
Fig.2. SEM image of a carbon micro-tube with a sub-micrometer diameter (sample taken from the sediment in water)
Fig.3. SEM image of a carbon micro-tube with a micrometer diameter (sample taken from the sediment in water)
Fig.4. SEM image of two carbon micro-tubes grown on the cathode.
4. Discussion of results From the experimental observations made above two types of the growth mechanisms can be imagined: First type [13] (see Figs.2-3): During the electrolysis Mg atoms are deposited on the surface of the graphite cathode. The Mg atoms intercalate into the spacing between the graphite planes. Then, due to different size of metal atoms compared to carbon atoms and different bonding types (metal-carbon compared with carbon-carbon) some stress between the graphite plates can take place, and this can lead to the ablation of the outside planes from the bulk of the cathode. However, the ablated planes have less thermodynamic stability suspended in the molten salts relative to their state in bulk graphite. The carbon atoms situated at the edge of the ablated planes with broken bonds start to rearrange themselves into a tube – into the structure with higher stability. The schematic explanation of this possibility can be seen in Fig.1, by steps 1 –1.a – 1.b. Second type (see Fig.4): The another concept of growth is the intercalation of the deposited metal atoms into the graphite. The stress caused by the different size and bonding of metal atoms and graphite planes causes partial separation of plates. The hanging plate, which is partly connected to the graphite cathode will be banded forming a tube. Later, due to further erosion of the cathode and/or the diffusion of metal atoms the tube can grow more. The schematic explanation of this possibility can be seen in Fig.1, steps2 – 2.a – 2.b. Obviously, the two mechanisms can take place at the same time as it happened in our experiments. In our further research the relative importance of the above two mechanisms will be studied in more details.
Conclusions Carbon microtubes have been successfully synthesized by an electrochemical way from NaCl – 5%MgCl2 molten salt at the surface of a graphite cathode. The length of the tubes are longer than 10 m m, while their diameter ranges between 0.2 m m and 2 m m. The wall thickness of the tubes is smaller than the diameter of the tube by about 10 times. Two different mechanism has been suggested for the growth of the carbon microtubes. Further research is planned to understand the factors affecting geometry and properties of carbon microtubes synthesized from molten salts.
Acknowledgements The authors are grateful to I.Sytchev for his help during the high-temperature experiments, and to dr. G.Kaptay for his discussions during this work.
References 1. S. Iijima, Nature, 354, (1991) 56 2. T.W.Ebbesen, Annu. Rev. Mater. Sci. 24 (1994) 235 3. Carbon nanotubes - Preparation and Properties – ed. by T.W.Ebbesen, CRC Press, Boca raton, FL, (1997) 4. M.H.Ge, K.Satler, Appl. Phys. Lett. 64 (1994) 710. 5. A.Thess, R.Lee, P.Nikolaev, H.J.Dai, P.Petit, J.Robert, C.H.Xu, Y.H.Lee, S.G.Kim, A.G.Rinzler, D.T.Colbert, G.E.Scuseria, D.Tomanek, J.E.Fischer, R.E.Smalley, Science, 273 (1996) 483 6. W.K.Hsu, J.P.Hare, M.Terrones, H.W.Kroto, D.R.M.Walton, P.J.F.Harris, Nature, 667 (1995) 687 7. W.K.Hsu, M.Terrones, J.P.Hare, H.Terrones, H.W.Kroto, D.R.M.Walton, Chemical Physics Letters 262 (1996) 161-166 8. G.Z.Chen, X.Fan, A.Luget, M.S.P.Shaffer, D.J.Fray, A.H.Windle: Journal of Electroanalytical Chemistry 446 (1998) 1-6 9. G.Z.Chen, I.Kinloch, M.S.P.Shaffer, D.J.Fray, A.H.Windle, in: “Molten Salts: from structural aspects to waste processing”, ed. by M. Gaune-Escard, begell house inc., NY,1999, 97-107 10. L.P.Bíró, J.Gyulai, G.I.Márk, C.S.Daróczi, Micron, 30 (1999) 245-254 11. L.P.Bíró, G.I.Márk, J.Gyulai, N.Rozlosnik, J.Kürti, B.Szabó, L.Frey, H.Ryssel, Carbon, 37 (1999) 739-744 12. J.Gyulai, Hungarian Chemical Journal, 56 (2001) 169-173 13. G.Kaptay, I.Sytchev, J.Miklósi, P.Nagy, P.Póczik, K.Papp, E.Kálmán, in: “Progress in Molten Salt Chemistry”, vol.1, ed. by R.W Berg and H.A.Hjuler, Elsevier, Paris, 2000, 257-262 14. J.Miklósi, P.Póczik, I.Sytchev, K.Papp, G.Kaptay, P.Nagy, E.Kálmán, Appl.Phys., A72 (2001) S189-S192. 15. J.Miklósi, P.Nagy, E.Kálmán, I.Sytchev, M.S.Yaghmaee, G.Kaptay, to be published in Proc. of MicroCAD, 2001.
|
||